A treatment that targets the immune system could help stop repeat heart attacks in people who have recently had one, according to the results of an early-stage clinical trial run in Cambridge. Two years after joining the trial, none of the participants that received treatment had further heart attacks.
Called aldesleukin, the treatment reduces inflammation in major blood vessels by boosting the activity of 'regulatory T cells', a part of the immune system. Aldesleukin is already used in much higher doses in the treatment of some kidney cancers.
Regulatory T cells, a unique type of white blood cell, are important gatekeepers of our immune system, and their discovery was recently awarded the 2025 Nobel Prize in Physiology or Medicine.
In the UK, someone is admitted to hospital every five minutes due to a heart attack. While seven out of ten people will survive a heart attack, many are left with damaged hearts, which can increase risk of further attacks.
People who have had a heart attack typically have high inflammation in major blood vessels and this is a strong indicator that they are at risk of further attacks.
Currently there is no approved treatment targeting inflammation in people following heart attacks.
Results published today (Thursday 8 January 2026) in Nature Medicine indicate that low-dose treatment with aldesleukin could prevent repeat heart attacks by reducing inflammation. The results arose from two related clinical trials known as IVORY and IVORY-FINALE, largely funded by the Medical Research Council.
Sixty patients who had recently had heart attacks took part in IVORY. Each was either given a low dose of aldesleukin or a placebo. Of these, 55 patients then participated in up to five years of follow-up health monitoring, known as IVORY-FINALE.
Participants had PET scans before and after treatment to assess inflammation in their blood vessels. On average, aldesleukin reduced inflammation by nearly 8%, with the greatest effect seen in blood vessels that initially had the highest levels of inflammation.
After two years follow-up, all patients treated with aldesleukin had no further heart attacks, while 11% of those treated with the placebo did. Larger studies are needed to confirm these findings before aldesleukin can be safely approved for widespread treatment of heart attack patients.
Mark's story:

One patient to have participated in the IVORY trial is Mark Andrews, 59, from Fulbourn, in Cambridgeshire.
In August 2022 he had a heart attack while cycling home from the gym. Despite being active, healthy and having no history of heart problems, tests at Addenbrooke’s Hospital confirmed that Mark was having a heart attack.
He was rapidly transferred here to Royal Papworth Hospital where a stent was fitted to hold open a narrowed artery, restoring blood flow to his heart. While still in hospital, Mark was invited to participate in the IVORY trial.
He said: “I was keen to join the trial. It wasn’t just the possibility that the treatment could stop me from having another heart attack. It was knowing the team would be closely monitoring my health, so they would probably spot it early if anything started to go wrong.”
Three years on from joining the trial, Mark has had no further heart attacks or symptoms. He works an IT specialist and still stays active including regular gym sessions.
He added, “The teams at Royal Papworth and Addenbrooke’s were fantastic. It’s a slow road to recovery and the contact with the teams as well as the specialist cardio rehab really helped to keep me active and get back to normal.”
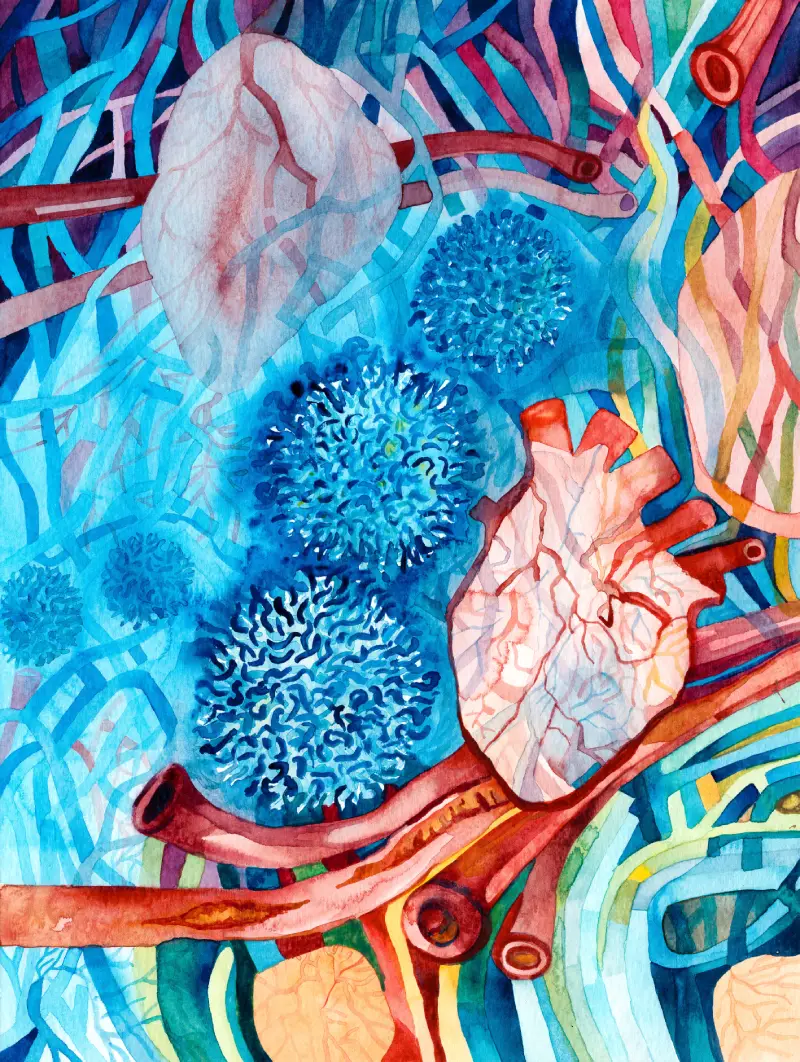
The research team commissioned this hand-painted watercolour illustration by Shaanea Mendis. It depicts regulatory t cells (tregs), pictured in blue, easing blood vessel inflammation and promoting healing after a heart attack through imagery and colour transition.
The IVORY and IVORY-FINALE trials were supported by the Medical Research Council, the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre, the NIHR Cambridge Clinical Research Facility and the British Heart Foundation.

Members of the IVORY research team
Professor James Rudd, Professor of Cardiovascular Medicine at the University of Cambridge, led the imaging aspects of the trial. He said: "It is very satisfying to see this type of advanced imaging test being used to identify a new treatment that could help reduce the risk of heart attacks in the future."
Professor Bryan Williams, Chief Scientific and Medical Officer at the British Heart Foundation, commented: “A treatment to reduce inflammation after a heart attack could be a game-changer and lead to a powerful treatment option for patients. It is exciting that aldesleukin, a drug already known to be safe, and used routinely, could potentially be repurposed to reduce the risk of future heart attacks. We look forward to seeing whether these results can be replicated in a larger study.”
Dr Adam Babbs, Associate Director of Translation at the Medical Research Council, which funded the trial, said, “By uncovering the complex biological mechanisms that trigger repeat heart attacks, researchers have identified a precision therapy targeting inflammation after an attack. It shows potentially life-saving promise for preventing repeat heart attacks.
“Repurposing an existing anti-cancer drug hugely speeds up progress to patient trials and could bring this treatment to the NHS much sooner if further studies to refine its use confirm its effectiveness in more patients.”
